Nuclear Envelope

The nuclear envelope has two membranes, each with the typical unit membrane structure. They enclose a flattened sac and are connected at the nuclear pore sites. The outermost membrane is continuous with the rough endoplasmic reticulum (ER) and has ribosomes attached (see figure to the left). The space between the outer and inner membranes is also continuous with rough endoplasmic reticulum space. It can fill with newly synthesized proteins just as the rough endoplasmic reticulum does. The nuclear envelope is enmeshed in a network of filaments for stability.

The nuclear envelope is shown in an electron micrograph in the figure to the right. The filaments outside the envelope are not visualized with these protocols. Also, the nuclear lamina just inside the nuclear envelope is not shown well (see paragraph below for description). However, one can see ribosomes on the outer membrane and the sac enclosed by the two membranes. Dense patches of Heterochromatin are seen just inside the inner membrane.
Nuclear lamina
The inner membrane of the nuclear envelope lies next to a layer of thin filaments which surrounds the nucleus except at the nuclear pores. These may also serve as stabilizing filaments. This structure is called the "nuclear lamina". It has the following structural and functional characteristics. Consists of "intermediate filaments", 30-100 nm thick.
These intermediate filaments are polymers of lamin, ranging from
60-75 kD
A-type lamins are inside, next to nucleoplasm; B-type lamins are
near the nuclear membrane (inner). They may bind to integral proteins inside that
membrane. The lamins may be involved in the functional organization of the
nucleus. They may play a role in assembly and disassembly before and after
mitosis. After they are phosphorylated, this triggers the disassembly of the lamina and
causes the nuclear envelope to break up into vesicles. Dephosphorylation reverses this and
allows the nucleus to reform.
See Alberts et al, Molecular Biology of the Cell, Garland Pub. 1994, p 567, Figure 12-18
NOTE: If antibodies to lamins are injected into cells, the nuclei cannot reform after cell division. Therefore, these lamins are vital to reassembly.
Return to MenuNuclear Pore Complex: Structure
Consult your text, Alberts et al., Molecular Biology of the Cell, Garland Pub., N.Y., Third Edition, 1994 pp 561-567 for more information and photos.
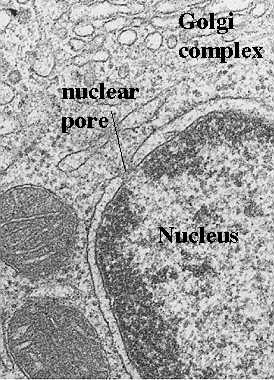
Nuclear pores are formed at sites where the inner and outer membranes of the nuclear envelope are joined. The figure to the left shows an electron micrograph of a nuclear pore. It appears as if the two membranes are pinched at that site, leaving a space filled with filamentous material. Sometimes a thin diaphragm may be seen running horizontally through the pore. Also, the chromatin which carries the genetic material is organized so that a space or "pathway" is created to the nuclear pore. A great deal of progress has been made in our understanding of how the pore is structured. However, we still do not know exactly how the components function. The following figure illustrates a model for the structure of the components of the nuclear pore complex. We will then show you how cytologists can visualize this structural organization in various preparations.

The above figure shows a view of the nuclear pore from the top. It contains 8 subunits that "clamp" over region of the inner and outer membrane where they join. Actually, they form a ring of subunits 15-20 nm in diameter. Each subunit projects a spoke-like unit into the center so that the pore looks like a wheel with 8 spokes from the top. Inside is a central "plug". The next (left) figure shows a cross section of the pore with the clamp-like complex adjacent to the membranes. The projected spoke is directed towards the central "plug' or granule.
Figure modified from Bloom and Fawcett, A Textbook of Histology, Chapter 1, Figure 1-11, Chapman and Hall Publishers, 1994
.

This electron micrograph shown in the figure to the right depicts a nuclear pore complex seen with the transmission electron microscope. As is obvious, little detail can be seen. The inner and outer membranes of the nuclear envelope are joined and there appears to be a diaphragm-like structure in the center. However, the intricate detail pictured in the foregoing figure cannot be appreciated. One needs to use different preparative techniques to see the subunits and their organization. These will be discussed and illustrated in the following sections.
How can you visualize the nuclear pore complex?
Negative Staining Technique
 One of the
techniques used to study nuclear pore complexes is called "negative staining".
This protocol deposits heavy metal stains around structures and delineates their surface
structure. When placed in an electron microscope, the heavy metal around the structure
retards the electron beam. The structure itself allows the electron beam to pass and this
activates the photographic emulsion. Thus, a "negative" image is created in the
photograph.
One of the
techniques used to study nuclear pore complexes is called "negative staining".
This protocol deposits heavy metal stains around structures and delineates their surface
structure. When placed in an electron microscope, the heavy metal around the structure
retards the electron beam. The structure itself allows the electron beam to pass and this
activates the photographic emulsion. Thus, a "negative" image is created in the
photograph.
The figure to the left illustrates a preparation of nuclear pore complexes that were isolated from an oocyte and spread on plastic. Then, the heavy metal stain was applied to delineate their structure. Note that one can visualize the 8 subunits, the spokes of the wheel and the central granule.
Figure modified from Bloom and Fawcett, A Textbook of Histology, Chapter 1, Figure 1-10, Chapman and Hall, Publishers, 1994
Freeze-fracture/freeze-etch
 Another way
of visualizing nuclear pores is via freeze-fracture/freeze
etch. This protocol involves the rapid freezing of structures followed by fracturing.
The membranes are cleaved along their lipid bilayer and
either the face next to the cytoplasm (protoplasmic or P face) or the extracellular (E)
face of the membrane is shown. Then, a replica is made of the membrane by evaporating
heavy metal over the surface. This replica is what is viewed in the transmission electron
microscope.
Another way
of visualizing nuclear pores is via freeze-fracture/freeze
etch. This protocol involves the rapid freezing of structures followed by fracturing.
The membranes are cleaved along their lipid bilayer and
either the face next to the cytoplasm (protoplasmic or P face) or the extracellular (E)
face of the membrane is shown. Then, a replica is made of the membrane by evaporating
heavy metal over the surface. This replica is what is viewed in the transmission electron
microscope.
The above figure shows a surface view of nuclear pores scattered in the inner nuclear envelope membrane. The subunits cannot be appreciated with this preparation. However, it can be used to study formation of nuclear pore complexes. This varies with the physiological state of the cell.
Figure modified from Bloom and Fawcett, A Textbook of Histology, Chapter 1, Figure 1-9, Chapman and Hall, Publishers, 1994

Another preparation shows more details of the structure of the nuclear pore complex. Here we see the subunits forming the rings and their spokes. Note that one of the pores appears to be open in the center, forming a channel.
Figure modified from Bloom and Fawcett, A Textbook of Histology, Chapter 1, Figure 1-9, Chapman and Hall, Publishers, 1994
The subunits also project fibrils from either side. At the nuclear side, these fibrils join to form a "nuclear cage" The fibrillar structures cannot be appreciated in any of the above micrographs. However the text shows the structures in a scanning electron micrograph (see Figure 12-10, Alberts et al, Molecular Biology of the Cell, Garland Pub., 1994)
Return to MenuHow does the nuclear pore complex work to transport material in and out of the nucleus?
The pore serves as a water filled channel and has an effective diameter of 10 nm.
Therefore, transport in and out of the nucleus can occur in several ways.
Diffusion
This can be tested by adding different sized molecules to the cytosol and watching the rate of transport of each group. For example, molecules of:
- 5,000 MW are freely diffusable
- 17,000 MW-- take 2 min to establish equilibrium
- 44,000 MW--take 30 min to establish equilibrium
- 60,000 MW--cannot move in by diffusion
This concept is important because it means that mature ribosomes (with both subunits joined) cannot reenter the
nucleus. Therefore, protein synthesis (translation of mRNA) must occur outside the
nucleus.
Active Transport
This form of transport is assumed when molecules larger than the pore diameter (10 nm) get into the nucleus. Studies with gold markers show that the pore can actually dilate up to 26 nm when it gets the appropriate signal. To understand the process, we need to answer the following questions.
- What is the signal?
- What tests can be used to prove a given signal allows transport?
- How does one prove that transport occurs via "active transport", i.e., requires energy or ATP)?
Before you continue, think about these questions.
- How would you find and test signals, cytochemically?
- How would you prove that the signal was valid?
- Finally, how would you prove that the process requires ATP?
In the following sections, we will illustrate experiments that were done to answer these questions.
What is the signal ?
Studies have shown that the signal is in the peptide sequences.
These are recognition sequences rich in lysine, arginine, and proline.
Signal may control direction of transport: Gold-labeled tRNA or 5S
RNA may leave the nucleus, but may not come back. Also, transport of RNA is inhibited by alteration of the 3' end or
the 5' cap structure.
What tests can be used to prove a particular signal?
 A peptide
sequence, called nucleoplasmin, was isolated and linked to colloidal gold. It was then
injected into an oocyte and traced with electron microscopy. As shown in the figure to the
left, the gold particles mark the site of transport of the nucleoplasmin and the studies
showed that it was transported into the nucleus. Small gold markers are evident inside the
nucleus.
A peptide
sequence, called nucleoplasmin, was isolated and linked to colloidal gold. It was then
injected into an oocyte and traced with electron microscopy. As shown in the figure to the
left, the gold particles mark the site of transport of the nucleoplasmin and the studies
showed that it was transported into the nucleus. Small gold markers are evident inside the
nucleus.
Figure was taken from Alberts et al., Molecular Biology of the Cell, Garland Pub., N.Y. 1994, Fig 12-15.
In another test, workers took advantage of the fact that nucleoplasmin has a head
region which is not transported into the nucleus and a tail region which is transported.
When the heads and tails were separated and each linked to gold, only the gold-labeled
tail region was transported. Gold-labeled head regions remained in the cytoplasm.

In a test of T antigens of the SV40 virus, if the sequence is altered by even one amino acid (see figure below), the peptide is no longer transported to the nucleus. This is shown in this figure. The studies used immunofluorescence to detect either the native or the altered sequences which were conjugated to fluorescein. Note that only the native peptide could enter the nucleus. In the above figure, the light, fluorescence micrograph on the left (A) shows entry of the native peptide into the ovoid nuclei. The micrograph on the right (B) shows no entry into nuclei. The altered peptide stays in the cytoplasm.
Figure was taken from Alberts et al., Molecular Biology of the Cell, Garland Pub., N.Y. 1994, Fig 12-13.
Finally, one can follow the exit of mRNAs by complementary DNA or RNA probes that
detect Poly (A)+ RNA. This probe can be linked to biotin, radioactive label, or
digoxygenin. It is then detected cytochemically.
How does one prove that transport requires energy (or ATP)?
Transport of mRNA can be inhibited by cooling the cells
(placing them at 4 C).
ATP Hydrolysis is required to import a protein into the nucleus.
In the absence of ATP, the proteins bind specifically at the cytosolic face of isolated
frog oocyte nuclei. When ATP is added, the proteins are allowed to enter. This can be
traced with colloidal gold labeling of the proteins. Studies show that ATP is needed for
entry, but not for binding to specific receptors.
For more information, contact:
Gwen Childs, Ph.D.,FAAA
Professor and Chair
Department of Neurobiology and Developmental Sciences
University of Arkansas for Medical Sciences
Little Rock, AR 72205
For questions, contact this email address:





