Receptor mediated endocytosis is a process by which cells internalize molecules or viruses. As its name implies, it depends on the interaction of that molecule with a specific binding protein in the cell membrane called a receptor. The following case will illustrate why you need to know about this clinically important cellular event. It is illustrated in the cartoon and electron micrographs in the side bar.
Study this unit and then return to the case with answers for Mr. Murphy.
Define major internalization routes:
The above figure diagrams the major internalization events. In the two views on the right, receptors are not needed for internalization. During phagocytosis, cells may simply internalize particles or cells, like bacteria (cell eating). In the second, called pinocytosis, cells internalize soluble material (cell drinking). In both types of internalization, the cells extend processes and bring cells or soluble material into the cell in a vacuole. In the presentation on lysosomes , we learned that the vacuole formed in the cell by phagocytosis or pinocytosis often became a lysosome after hydrolases were brought to it and the pH was adjusted. The vacuoles formed are called phagosomes or macropinosomes
Endosomes are formed by receptor-mediated endocytosis. In this case, cells bring in proteins and other types of ligands attached to the plasma membrane via receptors. The process depends first on specific binding to the receptor, which is a subject worthy of a lecture in itself. This figure shows this process as "coated pit endocytosis". The coated pit is a specialized region of the membrane that is coated with clathrin (for stability, to aid the transport process). The coated pit forms a coated vesicle and then loses its clathrin coat. It then joins with other coated pits to form a receptosome.
What types of ligands enter by receptor mediated endocytosis?
Toxins and lectins
- Diptheria Toxin
- Pseudomonas toxin
- Cholera toxin
- Ricin
- Concanavalin A
Viruses
- Rous sarcoma virus
- Semliki forest virus
- Vesicular stomatitis virus
- Adenovirus
Serum transport proteins and antibodies
- Transferrin
- Low density lipoprotein
- Transcobalamin
- Yolk proteins
- IgE
- Polymeric IgA
- Maternal IgG
- IgG, via Fc receptors
Hormones and Growth Factors
- Insulin
- Epidermal Growth Factor
- Growth Hormone
- Thyroid stimulating hormone
- Nerve Growth Factor
- Calcitonin
- Glucagon
- Prolactin
- Luteinizing Hormone
- Thyroid hormone
- Platelet Derived Growth Factor
- Interferon
- Catecholamines
How does the process work? An overview:
Receptors are brought to the plasma membrane by vesicles from the trans region of the Golgi complex . Review the definition of transmembrane proteins. Where and how are these receptor proteins inserted into the membrane? How does the Golgi complex maintain the fluidity of the plasma membrane, the receptors can move laterally in the membrane and collect in the specialized regions called clathrin coated pits.
When the ligand binds to its specific receptor, the ligand-receptor complex accumulates in the coated pits. In many cells, these pits and complexes begin to concentrate in one area of a cell. Cytochemically, this appears as patches of label on the cell surface (patching) Eventually, the patches coalesce to form a cap at one pole of the cell (capping) Not all cells form caps, but most do form patches. Why would this process be an advantage for the cells? Imagine the large amounts of extracellular fluid that would be taken up if the cells endocytosed the ligand receptor complex all over its surface. Thus, the pre-concentration process minimizes the amount of fluid that is taken up in the vesicle. Below is a micrograph showing the patching of receptors at one pole of the cell (Arrows, black labeling).
Effect of temperature on the process.
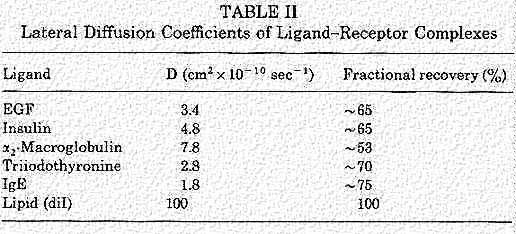
This table was taken from Endocytosis, Edited by Ira Pastan and Mark C. Willingham, Plenum Press, N.Y., 1985
To understand patching and capping, recall the studies of membranes and Membrane fluidity. Receptors are moving in the plane of the membrane as long as the temperature is 37 C. In the presentation on Membrane fluidity, we talked about photobleaching with a laser beam. This allows you to study the lateral diffusion coefficients of the ligand-receptor complexes. Fluorescent molecules signal the site of the complexes and they are bleached after the laser exposure. Then, the photobleaching system measures the speed of the recovery in the bleached area (return of the fluorescence) as the label returns by lateral diffusion.
An example of some measurements for different receptors is found in this table. The objective of the lateral movement is to collect the ligand-receptor complex in the clathrin coated pits. So, some receptors appear to be moving faster than others. One might speculate that this may be related to size of the receptor or the ligand.

Temperature may affect the binding of the ligand (rate) as well as the lateral mobility of the ligand-receptor complex. Some ligands will not bind well at low temperatures. However, others will bind, but not be taken in. This photograph shows the peroxidase (HRP) detection of a ligand that is distributed on the membrane at 4 C. Note the right hand control panel that shows absence of label in the presence of competing unlabeled ligand. Note the presence of the coated pit, even in the control. So, the formation of these is not temperature dependent. However, after warming for a few minutes, the formation of vesicles and endosomes is evident. It is important to note that Receptor mediated endocytosis is much faster than phagocytosis or pinocytosis. If one were to simply have a non-binding ligand present, it might take hours for the ligand to enter via pinocytosis. Thus, this rapid uptake coupled with the absence of label in the presence of competing ligand is a sign that this is receptor mediated.
This figure was taken from Endocytosis, Edited by Ira Pastan and Mark C. Willingham, Plenum Press, N.Y., 1985

Some coated vesicles may be configured with a deep invagination, called a "neck". These were discovered by Willingham and Pastan and can be seen in serial sections as a thin region connection between the outside of the membrane and the vesicle. The vesicle contains labeled ligand attached to receptor. Formation of these necks is definitely temperature dependent as can be seen in the following table.
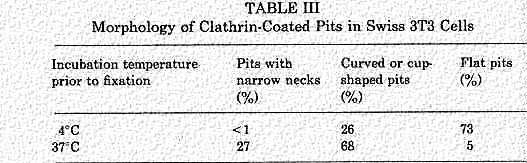
This figure and Table were taken from Endocytosis, Edited by Ira Pastan and Mark C. Willingham, Plenum Press, N.Y., 1985
Return to Menu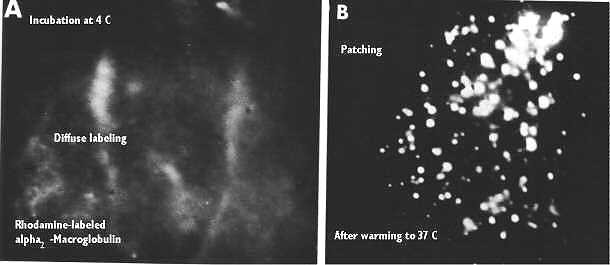
Finally, temperature is important to the overall patching and capping process discussed during the lecture on membranes. There we showed that, after they are bound, Membrane Receptors move laterally in the plane and groups of receptor-ligand complexes may actually coalesce in a patch and eventually in a cap. Antibodies are good examples of receptors that react this way. The left hand figure (above) shows what happens if the temperature is 4 C. There is a diffuse labeling. Warming the cells immediately produces patching.
This figure was taken from Endocytosis, Edited by Ira Pastan and Mark C. Willingham, Plenum Press, N.Y., 1985
Formation of Cathrin Coated Pits
Clathrin coats surround the pit as diagrammed in the above cartoon. The assembly of the clathrin molecules on the pit appears to drive the pit to invaginate. This cage-like molecule may help stabilize the vesicle as it buds from the membrane.
Clathrin coated pits may move in the plane of the membrane, however recent studies show that there is an "organized movement" as if the pits are tethered to cytoskeletal elements. The following paper studies coated pits in living cells that were transfected with a plasmid carrying a cDNA for green fluorescent protein (GFP) attached to the light chain of clathrin. Gaidarov I, Santini, F, Warren, RA and Keen, JH Spatial control of coated-pit dynamics in living cells. Nature, Cell Biology 1: 1-7. 1999.
The cells made GFP-clathrin and were able to insert the protein into coated pits. This was tested via antibodies to coated pit proteins as well as studies of the endocytosis of transferrin. When time lapse photography was used to learn if the coated pits moved, they found that the pits appeared and disappeared at intervals. Studies of regional spacing showed that appearance of new pits was often close to sites of old pits, suggesting regional organization. Superimposed images showed a linear pattern as if the pits were organized.
The studies showed that the coated pits were resistent to detergents (Triton-X). And, they were able to show that the retraction of cellular processes that followed triton-X treatment produced linear movement of each coated pit in the plasma membrane as if it was organized by or on cytoskeletal elements. See the paper by this group (above citation) for the photographs and movies of these findings.
Role of beta-Arrestins in guiding receptors to the clathrin-coated pits.
Beta-arrestin forms a complex with Adaptin (AP-2) and clathrin to both guide the receptor into the pit and keep it there.
Can more than one receptor type enter a vesicle?
Supposing a cell is stimulated with two hormones (and it has receptors for both), or a hormone and a growth factor. Do both ligands enter via the same packages, or is there a selection for one particular type of ligand in a coated pit. This is a perfect question for cytochemistry with different types of labels attached to different ligands. For example, one can use colloidal gold, ferritin, peroxidase and detect as many as 2-4 ligands on a given cell.
The following figure shows that multiple ligands can enter the cell in the same coated pit. Furthermore, the vesicles will carry them to the same receptosomes. The photo shows co-detection of ligands as diverse as Epidermal growth factor, vesicular stomatitis virus, or alpha 2 macroglobulin. Labeling molecules (signalling molecules) included gold, peroxidase, ferritin, or the virus itself. This figure was taken from Endocytosis, Edited by Ira Pastan and Mark C. Willingham, Plenum Press, N.Y., 1985

Formation of Endocytic vesicles.
Once the vesicle has formed, the clathrin coat is lost (perhaps via a chaperone protein of the heat shock protein 70 family). The loss of the coat is an energy requiring process. After the coat is lost, the vesicles join with other vesicles to form endosomes or receptosomes. The following electron micrograph shows clathrin coated pits forming a vesicle. It is taking up lipoprotein particles. Note how thick and well defined the clathrin coat is.
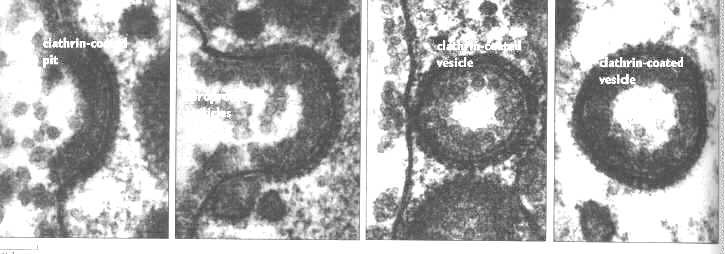
This micrograph was taken from Endocytosis, Edited by Ira Pastan and Mark C. Willingham, Plenum Press, N.Y., 1985
Low Density Lipoprotein Receptors are a good example of receptor-mediated endocytosis.
LDL carries cholesterol to the cells and bind specific receptors which undergo receptor mediated endocytosis to provide an important source of cholesterol for all cells. LDL is considered the “bad cholesterol”, only if it fails to get into the cell and ends up in the blood stream. How could that happen? There are clinical conditions in which the LDL receptors are defective. The discovery of this clinically important system resulted in Nobel Prizes for Brown and Goldstein!!
Now, you can solve Mr. Murphy’s case!! The important thing to remember is the type of defect. It is in the LDL receptor! This is often a question on boards and you may have to identify which molecule is defective from a selection, including: AP-2, Adaptin, clathrin, LDL, LDL receptor, Arrestin, etc. So, if you know the function of each of the players, you can answer the Board questions accurately. Go over the process of internalization until you know the steps and each of the molecules involved.
Can You Distinguish profiles showing exocytosis and
endocytosis?
Clathrin coated pits serve like a flat "basket", stabilizing the area to be internalized. They are not exclusive to the plasma membrane. However, it is important to be able to distinguish them from exocytosis profiles associated with the plasma membrane which serve as the secretory route for secreted products such as hormones, neurotransmitters, growth factors, etc.

For example, the above electron micrograph is showing the process of exocytosis . The process begins by fusion of the membranes at the peripheral pole of the granule. Then an opening is created which widens to look like an omicron figure. This opening allows the granular material to be released. The membrane is now part of the plasma membrane and any proteins carried with it can be incorporated into the plasma membrane. Note that there is no coating on the membrane. This figure was taken from Alberts et al, Molecular Biology of the Cell, Garland Publishing Third Edition, 1994

Receptor Mediated Endocytosis
In contrast, this micrograph shows a figure which looks something like an omicron, however, this view is showing receptor mediated endocytosis of virus particles. In both cases, the membrane is coated with clathrin and these represent classical receptor mediated endocytosis profiles. Most ligands cannot be visualized by themselves, like a virus particle. Therefore, the cytochemist must attach label to the ligand. Alternatively, the cytochemist could immunocytochemically detect the receptor with antibodies that recognize the extracellular domain. This figure was taken from Endocytosis, Edited by Ira Pastan and Mark C. Willingham, Plenum Press, N.Y., 1985

Continue studies of Receptor Mediated Endocytosis to learn what happens after Internalization!
For more information, contact:
Gwen Childs, Ph.D., FAAA
Professor and Chair
Department of Neurobiology and Developmental Sciences
University of Arkansas for Medical Sciences
Little Rock, AR 72205
For questions, contact this email address:




